GUIDELINES PROVIDE A RISK-STRATIFIED FRAMEWORK FOR TREATMENT
The NMIBC treatment algorithm below is adapted from the American Urological Association (AUA)/Society of Urologic Oncology (SUO) and NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®).
AUA/SUO Guideline Strong Recommendation;
Evidence Strength: Grade B1
In a high-risk patient with newly diagnosed CIS, high-grade T1, or high-risk Ta urothelial carcinoma, a clinician should administer a 6-week induction course of Bacillus Calmette–Guérin (BCG).
AUA/SUO Guideline Moderate Recommendation;
Evidence Strength: Grade B1
In a high-risk patient who completely responds to induction BCG, a clinician should continue maintenance BCG, based on availability, for three years, as tolerated.
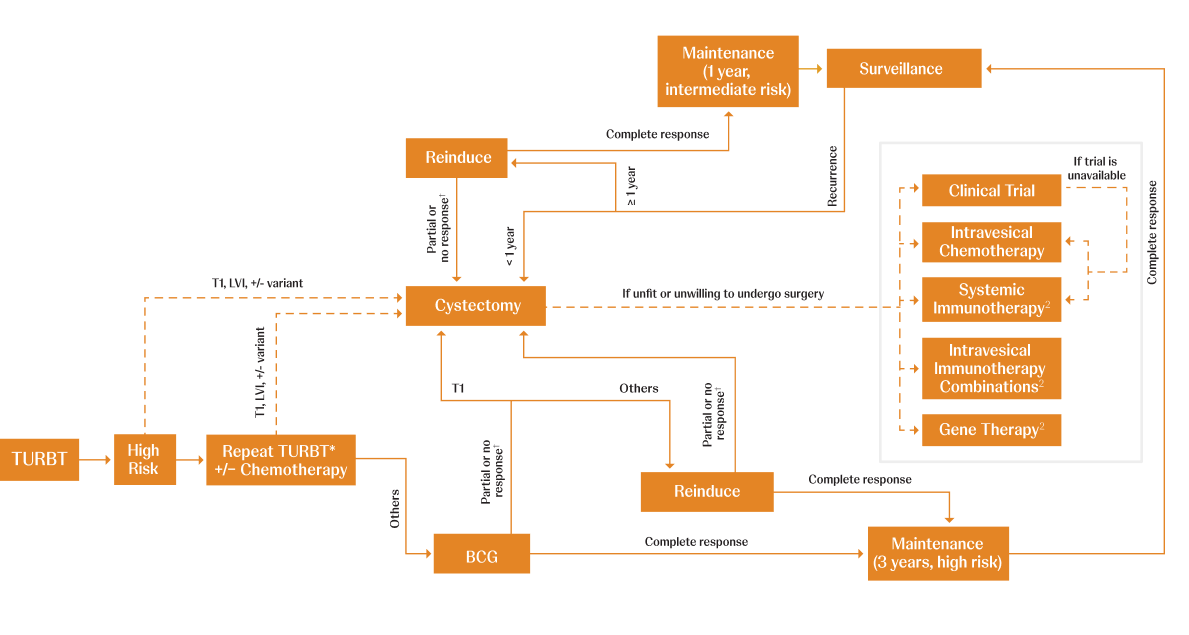
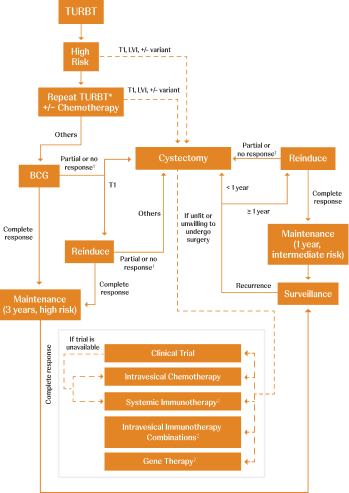
Adapted from AUA/SUO NMIBC Treatment Algorithm.
CIS=carcinoma in situ; LVI=lymphovascular invasion; TURBT=transurethral resection of bladder tumor.
*Timely repeat TURBT (within 6 weeks) should be performed if there are concerns regarding an incomplete resection and/or if bladder-sparing treatment (eg, intravesical therapy or surveillance) is being planned.1
†BCG unresponsiveness indicates that a patient didn’t respond to an adequate dose of BCG therapy. Adequate BCG is defined as 5 to 6 initial doses in addition to 2 to 3 maintenance doses. If a patient doesn’t respond after this, it is determined that no further doses will be beneficial.3,4
References:
Holzbeierlein JM, Bixler BR, Buckley DI, et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO Guideline: 2024 amendment. J Urol. 2024;211(4):533-538. doi:10.1097/ju.0000000000003846
Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Bladder Cancer V7.2024. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed March 10, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org.
Kim HS, Seo HK. Emerging treatments for bacillus Calmette-Guérin-unresponsive non-muscle-invasive bladder cancer. Investig Clin Urol. 2021;62(4):361-377. doi:10.4111/icu.20200602
Kamat AM. Risk stratification, definitions of disease states & BCG unresponsive disease. Presented at: Food & Drug Administration workshop;2021.
NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
AUA/SUO Guideline Moderate Recommendation;
Evidence Strength: Grade B1
In an intermediate-risk patient, a clinician should consider administration of a 6-week course of induction intravesical chemotherapy or immunotherapy.
AUA/SUO Guideline Moderate Recommendation;
Evidence Strength: Grade C1
In an intermediate-risk patient who completely responds to induction BCG, a clinician should consider maintenance BCG for 1 year, as tolerated.
AUA/SUO Guideline Conditional Recommendation;
Evidence Strength: Grade C1
In an intermediate-risk patient who completely responds to an induction course of intravesical chemotherapy, a clinician may utilize maintenance therapy.
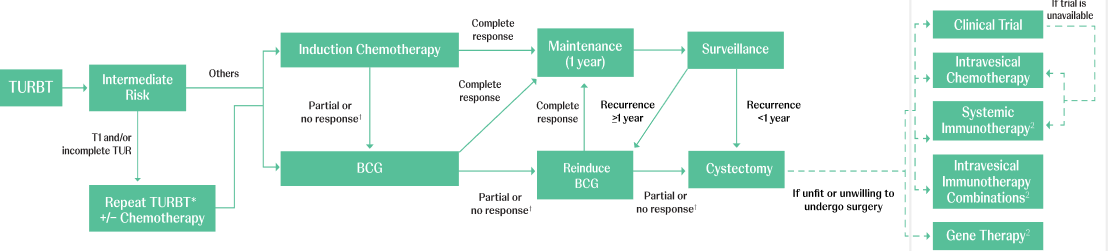
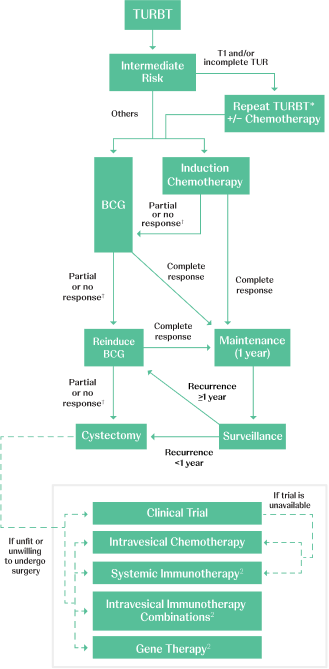
Adapted from AUA/SUO NMIBC Treatment Algorithm.
BCG=Bacillus Calmette–Guérin; TURBT=transurethral resection of bladder tumor.
*Timely re-TURBT (within 6 weeks) should be performed if there are concerns regarding an incomplete resection and/or if bladder-sparing treatment (eg, intravesical therapy or surveillance) is being planned.1
†BCG unresponsiveness indicates that a patient didn’t respond to an adequate dose of BCG therapy. Adequate BCG is defined as 5 to 6 initial doses in addition to 2 to 3 maintenance doses. If a patient doesn’t respond after this, it is determined that no further doses will be beneficial.3,4
References:
Holzbeierlein JM, Bixler BR, Buckley DI, et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO Guideline: 2024 amendment. J Urol. 2024;211(4):533-538. doi:10.1097/ju.0000000000003846
Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Bladder Cancer V7.2024. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed March 10, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org.
Kim HS, Seo HK. Emerging treatments for bacillus Calmette-Guérin-unresponsive non-muscle-invasive bladder cancer. Investig Clin Urol. 2021;62(4):361-377. doi:10.4111/icu.20200602
Kamat AM. Risk stratification, definitions of disease states & BCG unresponsive disease. Presented at: Food & Drug Administration workshop;2021.
NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
AUA/SUO Guideline Moderate Recommendation;
Evidence Strength: Grade B1
In a patient with suspected or known low- or intermediate-risk bladder cancer, a clinician should consider administration of a single postoperative instillation of intravesical chemotherapy within 24 hours of TURBT. In a patient with suspected perforation or extensive resection, a clinician should not use postoperative intravesical chemotherapy.
AUA/SUO Guideline Moderate Recommendation;
Evidence Strength: Grade C1
In a low-risk patient, a clinician should not administer induction intravesical therapy.
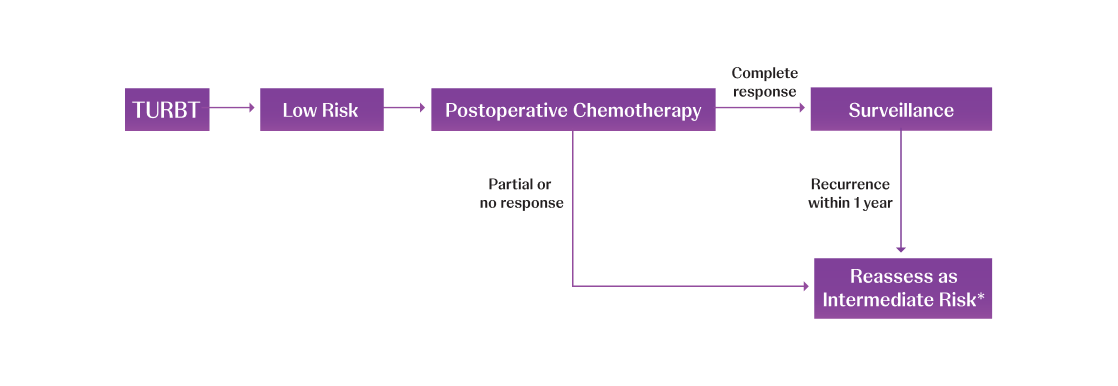
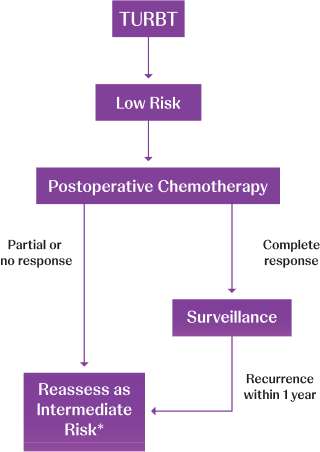
Adapted from AUA/SUO NMIBC Treatment Algorithm.
TURBT=transurethral resection of bladder tumor.
*Consider fulguration in low-volume disease recurrence; otherwise reassess as intermediate risk.1
References:
Holzbeierlein JM, Bixler BR, Buckley DI, et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO Guideline: 2024 amendment. J Urol. 2024;211(4):533-538. doi:10.1097/ju.0000000000003846
Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Bladder Cancer V7.2024. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed March 10, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org.
NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.